Sotorasib
Sotorasib (pINN), codenamed AMG 510, is an experimental cancer drug. It targets a specific mutation, G12C, in the protein KRAS which is responsible for various forms of cancer.[1][2]
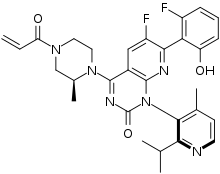 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C30H30F2N6O3 |
| Molar mass | 560.606 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Clinical development
Sotorasib is being developed by Amgen. Phase 1 clinical trials were completed in late 2020.[3][4][5] In December 2019, it was approved to begin Phase 2 clinical trials.[6]
Because the G12C KRAS mutation is relatively common in some cancer types, 14% of non-small-cell lung cancer adenocarcinoma patients and 5% of colorectal cancer patients,[7] and sotorasib is the first drug candidate to target this mutation, there have been high expectations for the drug.[7][8][9] The Food and Drug Administration has granted a fast track designation to sotorasib for the treatment of metastatic non-small-cell lung carcinoma with the G12C KRAS mutation.[10]
Chemistry and pharmacology
Sotorasib can exist in either of two atropisomeric forms and one is more active than the other.[7] It selectively forms an irreversible covalent bond to the sulfur atom in the cysteine residue that is present in the mutated form of KRAS, but not in the normal form.[7]
See also
- Adagrasib
References
- "KRAS mutant-targeting AMG 510". NCI Drug Dictionary. National Cancer Institute. 2011-02-02. Retrieved November 16, 2019.
- Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. (November 2019). "The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity". Nature. 575 (7781): 217–223. Bibcode:2019Natur.575..217C. doi:10.1038/s41586-019-1694-1. PMID 31666701.
- Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. (2020). "KRASG12C inhibition with sotorasib in advanced solid tumors". N Engl J Med. doi:10.1056/NEJMoa1917239. PMC 7571518.
- Clinical trial number NCT03600883 for "A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of AMG 510 in Subjects With Solid Tumors With a Specific KRAS Mutation " at ClinicalTrials.gov
- "The Discovery Of Amgen's Novel Investigational KRAS(G12C) Inhibitor AMG 510 Published In Nature" (Press release). Amgen. October 30, 2019. Retrieved November 16, 2019.
- Irving M (2019-12-24). "Drug targeting common cancer cause enters phase 2 clinical trials". New Atlas. Retrieved 2019-12-24.
- Halford B (April 3, 2019). "Amgen unveils its KRas inhibitor in human clinical trials: AMG 510 shuts down a mutant version of the cancer target via covalent interaction". Chemical & Engineering News. 97 (4). Retrieved November 16, 2019.
- Al Idrus A (September 9, 2019). "Amgen's KRAS drug continues to deliver but faces 'curse' of high expectations". fiercebiotech.com. Retrieved November 16, 2019.
- Kaiser J (2019-10-30). "Two new drugs finally hit 'undruggable' cancer target, providing hope for treatments". Science Magazine. AAAS. Retrieved November 16, 2019.
- Astor L (September 9, 2019). "FDA Grants AMG 510 Fast Track Designation for KRAS G12C+ NSCLC". targetedonc.com. Retrieved November 16, 2019.