Daunorubicin/cytarabine
Liposomal daunorubicin/Cytarabine is a fixed-dose combination of liposomal bound cytarabine and daunorubicin that delivers the two medications in a 5:1 molar ratio.
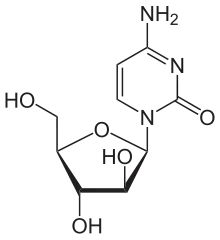 Cytarabine | |
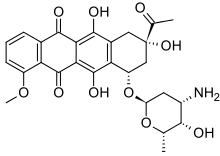 daunorubicin | |
| Combination of | |
|---|---|
| Cytarabine | Antimetabolite |
| daunorubicin | Anthracycline |
| Clinical data | |
| Trade names | Vyxeos |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| KEGG | |
It is used in the treatment of acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC).[1][2]
References
- "Vyxeos (cytarabine/daunorubicin liposomal) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 2019-03-19.
- "Vyxeos (cytarabine and daunorubicin) FDA Approval History". Drugs.com. Retrieved 2018-12-30.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.