Kappadione
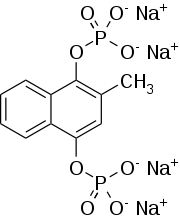
Kappadione is a Vitamin K derivative. It was manufactured by Eli Lilly and Company.[1] Chemically, it is menadiol sodium diphosphate. It was previously approved by FDA prior to 1982 and marketed by Lilly Marketing. This has been discontinued and is not available in North America.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.558 |
| Chemical and physical data | |
| Formula | C11H8Na4O8P2 |
| Molar mass | 422.084 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- "Kappadione overview, medicine.org". Archived from the original on 2016-03-03. Retrieved 2008-04-14.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.