Netarsudil
Netarsudil, sold under the brand name Rhopressa among others, is a medication for the treatment of glaucoma. In the United States in December 2017, the Food and Drug Administration (FDA) approved a 0.02% ophthalmic solution for the lowering of elevated intraocular pressure in people with open-angle glaucoma or ocular hypertension.[2][3] It targets the trabecular meshwork directly.[4]
 | |
| Clinical data | |
|---|---|
| Pronunciation | ne TAR soo dil |
| Trade names | Rhopressa, Rhokiinsa |
| Other names | AR-11324 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618014 |
| License data |
|
| Routes of administration | Eye drops, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
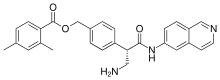
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.251.524 |
| Chemical and physical data | |
| Formula | C28H27N3O3 |
| Molar mass | 453.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The FDA considers it to be a first-in-class medication.[5]
References
- "Rhokiinsa EPAR". European Medicines Agency (EMA). 16 September 2019. Retrieved 27 September 2020.
- "Rhopressa (netarsudil) Ophthalmic Solution". U.S. Food and Drug Administration (FDA). 29 January 2018. Retrieved 3 June 2020.
- "Aerie (AERI) Gets Early FDA Approval for Lead Drug Rhopressa". 19 December 2017.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
External links
- "Netarsudil". Drug Information Portal. U.S. National Library of Medicine.
- "Netarsudil mesylate". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.