Voclosporin
Voclosporin, sold under the brand name Lupkynis, is a calcineurin inhibitor used as an immunosuppressant medication.[1] It was approved by the U.S. Food and Drug Administration (FDA) for the treatment of lupus nephritis (LN) on January 22, 2021.[2][3][4]
 | |
| Names | |
|---|---|
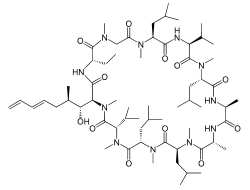
| IUPAC name
(3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-Ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methyl-4,6-heptadien-1-yl]-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone | |
| Other names
VCS, ISA247, Luveniq | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C63H111N11O12 |
| Molar mass | 1214.646 g·mol−1 |
| Pharmacology | |
| L04AD03 (WHO) | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Clinical data | |
|---|---|
| Trade names | Lupkynis |
| License data |
|
| Identifiers | |
| DrugBank | |
| KEGG | |
It is an analog of ciclosporin that has enhanced action against calcineurin and greater metabolic stability.[5] Voclosporin was discovered by Robert T. Foster and his team at Isotechnika in the mid 1990s.[6] Isotechnika was founded in 1993 and merged with Aurinia Pharmaceuticals in 2013. In Jan. 2021, Aurinia Pharmaceuticals received approval from the Food & Drug Administration to sell the drug Lupkynis.
Initially, voclosporin was a mixture of equal proporations of cis and trans geometric isomers of amino acid-1 modified cyclosporin. Later, in collaboration with Roche in Basel, Switzerland, voclosporin's manufacturing was changed to yield the predominantly trans isomer which possesses most of the beneficial effect of the drug (immunosuppression) in the treatment of organ transplantation and autoimmune diseases.
References
- Sin FE, Isenberg D (October 2018). "An evaluation of voclosporin for the treatment of lupus nephritis" (PDF). Expert Opinion on Pharmacotherapy. 19 (14): 1613–1621. doi:10.1080/14656566.2018.1516751. PMID 30207816. S2CID 52196375.
- "Voclosporin: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 25 January 2021.
- "FDA Approves Aurinia Pharmaceuticals' Lupkynis (voclosporin) for Adult Patients with Active Lupus Nephritis". Aurinia Pharmaceuticals Inc. (Press release). 22 January 2021. Retrieved 25 January 2021.
- "Drug Trials Snapshot: Lupkynis". U.S. Food and Drug Administration (FDA). 22 January 2021. Retrieved 12 February 2021.
- "What is voclosporin?". Isotechnika. Retrieved October 19, 2012.
- U.S. Patent 6,605,593
External links
- "Voclosporin". Drug Information Portal. U.S. National Library of Medicine.