Voglibose
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus.[1] Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Voglibose was first launched in 1994, under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.[2]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
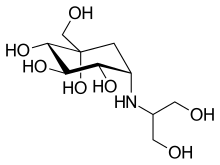
| Formula | C10H21NO7 |
| Molar mass | 267.278 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Postprandial hyperglycemia (PPHG) is primarily due to first phase insulin secretion. Alpha glucosidase inhibitors delay glucose absorption at the intestine level and thereby prevent sudden surge of glucose after a meal.
There are three drugs which belong to this class, acarbose, miglitol and voglibose, of which voglibose is the newest.
References
- Chen X, Zheng Y, Shen Y (2006). "Voglibose (Basen, AO-128), one of the most important alpha-glucosidase inhibitors". Current Medicinal Chemistry. 13 (1): 109–16. doi:10.2174/092986706789803035. PMID 16457643.
- "Voglibose". AdisInsight. Springer Nature Switzerland AG.
Further reading
- Greenstein B (2004). Clinical Pharmacology for nurses (17th ed.). Elsevier Limited, Churchill Livingstone.