Clofedanol
Clofedanol (INN) or chlophedianol (BAN) is a centrally acting cough suppressant used in the treatment of dry cough. Clofedanol has local anesthetic, antispasmodic, and antihistamine properties,[1] and may have anticholinergic effects at high doses.[2] It is marketed in Canada under the trade name Ulone. GM Pharmaceuticals owns the patents to 113 combinations with Chlophedianol and was the first company to launch the cough suppressant in the United States.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Ulo, Ulone, Coldrin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.219 |
| Chemical and physical data | |
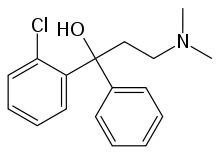
| Formula | C17H20ClNO |
| Molar mass | 289.80 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chlophedianol was approved for OTC status in 1987 by the FDA OTC monograph process[4] and its safety and efficacy data are limited. It was formerly sold over-the-counter in the United States under the trade name Ulo, as a syrup with a dosage of 25 mg/5 mL; however, it has been withdrawn from the market.[5]
Unlike many other antitussive drugs such as dextromethorphan, it binds poorly to the sigma-1 receptor.[6]
It is sold in Japan as an over-the-counter drug under the name Coldrin.[7]
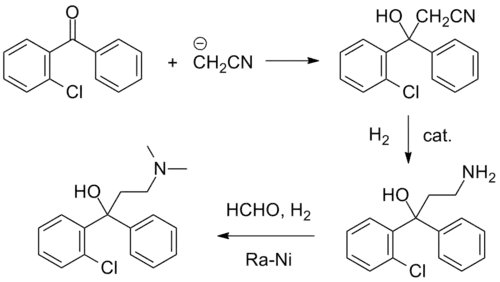
Synthesis
See also
References
- Martín, Alfonso Velasco (2004). "Tratamiento sintomático de la tos y del resfriado común". Farmacología clínica y terapéutica médica. p. 260. ISBN 9788448604271.
- "Clofedanol" (in French). BIAM. 1998-07-24. Retrieved 2007-04-15.
- "How Odes Mitchell Built a Pharmaceutical Empire". D Magazine. Retrieved 2020-11-25.
- "Department of Health and Human Services. Food and Drug Administration. 21 CFR Parts 310, 341, and 369. Docket No. 76N-052T. Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the-counter human use; final monograph for OTC antitussive drug products. Federal Register 1987;52(155):30042-57" (PDF). FDA.gov. 1987-08-12.
- Approved Drug Products with Therapeutic Equivalence Evaluations, March 2020 Edition (Report). Food and Drug Administration. March 20, 2020. Retrieved April 7, 2021.
- Klein M, Musacchio JM (October 10, 1988). "Dextromethorphan binding sites in the guinea pig brain". Cellular and Molecular Neurobiology. 8 (2): 149–156.
- "クロフェダノール:コルドリン" [Clofedanol: Coldrin]. Medicine 110 (in Japanese). Retrieved March 22, 2021.