Pirlimycin
Pirlimycin hydrochloride belongs to the lincosamide class of antimicrobials. Under the trade name Pirsue,[1] it is used in the treatment of mastitis in cattle.
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intramammary |
| ATCvet code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
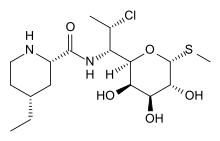
| Formula | C17H31ClN2O5S |
| Molar mass | 410.95 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Activity
Pirlimycin is active against Gram-positive bacteria, specifically Staphylococcus aureus and coagulase negative species of Staphylococcus and Streptococcus. It has no activity against Gram-negative bacteria.[2]
Mechanism of action
It is bacteriostatic and acts by inhibiting bacterial protein synthesis via binding with the 50S subunit of the ribosome.
References
- Pirsue Sterile Solution
- "USP: Pirlimycin" (PDF). Archived from the original (PDF) on 2009-05-09. Retrieved 2009-06-28.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.