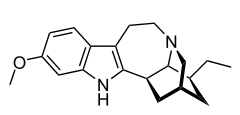
Tabernanthine
Tabernanthine is an alkaloid found in Tabernanthe iboga.[1]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H26N2O |
| Molar mass | 310.441 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It has been used in laboratory experiments to study how addiction affects the brain.[2]
Tabernanthine persistently reduced the self-administration of cocaine and morphine in rats.[3]
Pharmacology
It is NMDA receptor (Ki = 10.5 μM) antagonist.[4] Compared to ibogaine, it binds weakly to σ1 and σ2 receptor.[4]
See also
- Coronaridine
- Ibogaine
- Ibogamine
- Voacangine
References
- Bartlett, M. F.; Dickel, D. F.; Taylor, W. I. (1958). "The Alkaloids of Tabernanthe iboga. Part IV.1 The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine". Journal of the American Chemical Society. 80: 126–136. doi:10.1021/ja01534a036.
- Levi MS, Borne RF (October 2002). "A review of chemical agents in the pharmacotherapy of addiction". Curr. Med. Chem. 9 (20): 1807–18. doi:10.2174/0929867023368980. PMID 12369879.
- Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW Jr, Carlson JN (September 1994). "Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum". Brain Res. 657 (1–2): 14–22. doi:10.1016/0006-8993(94)90948-2. PMID 7820611.
- Christophe Wiart (16 December 2013). Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. pp. 67–69, 73. ISBN 978-0-12-398383-1.
Treatment of drug dependence (N07B) | |
|---|---|
| Nicotine dependence | |
| Alcohol dependence | |
| Opioid dependence | |
| Benzodiazepine dependence | |
| Research | Salvia divinorum |
| AMPAR |
|
|---|---|
| KAR |
|
| NMDAR |
|
| |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.