Dexelvucitabine
Dexelvucitabine is a failed experimental agent for the management of HIV infection. Dexelvucitabine is a cytidine nucleoside analog and nucleoside reverse transcriptase inhibitor. It was found to inhibit HIV-1 replication in vitro and during Phase II clinical trials, it was found to decrease mean viral load in patients with HIV.
 | |
| Names | |
|---|---|
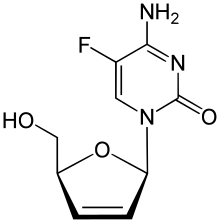
| IUPAC name
4-Amino-5-fluoro-1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]pyrimidin-2-one | |
| Other names
Reverset | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H10FN3O3 |
| Molar mass | 227.195 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
On April 3, 2006, Pharmasset and Incyte, the pharmaceutical companies developing dexelvucitabine announced the decision to cease further trials and development of the drug due to an increased incidence of grade 4 hyperlipasemia (an excess of the pancreatic enzyme, lipase) in a phase II trial.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.