Pipazetate
Pipazetate (INN) (brand names Dipect, Lenopect, Selvigon, Theratuss, Toraxan), or pipazethate (USAN), is a pyridobenzthiazine cough suppressant, closely related to the phenothiazine class.[1][2] It binds to the sigma-1 receptor with an IC50 value of 190 nM.[3] It also has local anesthetic action, and in large doses can produce seizures.[4]
 | |
| Clinical data | |
|---|---|
| Trade names | Dipect, Lenopect, Selvignon, Selvigon, Theratuss, Toraxan |
| Other names | Pipazethate; D-254; LG-254; SKF-70230A; SQ-15874 |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.826 |
| Chemical and physical data | |
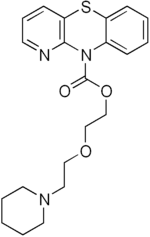
| Formula | C21H25N3O3S |
| Molar mass | 399.51 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
As the brand name Theratuss, it was withdrawn from the US market in 1972 when the manufacturer, E.R. Squibb and Sons, failed to produce evidence of efficacy.[5] Clinical studies showed that it did not decrease cough frequency at recommended dosages.[6]
Side effects, which are infrequent, include nausea, vomiting, drowsiness, fatigue, rash, and tachycardia.[6]
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 985–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1418–. ISBN 978-3-88763-075-1.
- Klein M, Musacchio JM (October 10, 1988). "Dextromethorphan binding sites in the guinea pig brain". Cellular and Molecular Neurobiology. 8 (2): 149–156. doi:10.1007/BF00711241. PMID 3044591. S2CID 33844132.
- Martín, Alfonso Velasco (2004). "Tratamiento sintomático de la tos y del resfriado común". Farmacología clínica y terapéutica médica. p. 259. ISBN 9788448604271.
- Certain Preparations Containing Dihyprylone or Pipazethate Hydrochloride; Notice of Withdrawal of Approval of New-Drug Applications (PDF). Federal Register (Report). 37. August 5, 1972. p. 15887. FDC–D–458.
- Council on Drugs (1971). AMA Drug Evaluations (Report). Chicago: American Medical Association. p. 360–3. LCCN 75147249. Retrieved April 5, 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.